Haber Process Pdf
The Haber process in the past more often referred to as the Haber-Bosch process is one of the towering achievements of industrial chemistry. In addition to the chemical reaction equilibrium Haber recognized that reaction rate was a determining factor.
Https Link Springer Com Content Pdf 10 1007 978 1 4020 3611 8 2 Pdf
The process combines nitrogen from the air with hydrogen derived mainly from natural gas methane into ammonia.
Haber process pdf. The Haber Processpdf - The Haber Process History u2022 Fritz Haber 1909 u2013 first to produce ammonia from nitrogen air and hydrogen natural gas u2022 Used The Haber Processpdf - The Haber Process History u2022. By convention DH refers to the forward reaction which is exothermic in this case. It is reacted with steam to form hydrogen.
It has enabled us to produce enough nitrogen fertiliser to feed the current world population to provide enough explosive to fight as many minor wars as seems desirable to desperate enough people and to be able to do this for the. 2019-04-10 Updated April 10 2019 The Haber-Bosch process is a process that fixes nitrogen with hydrogen to produce ammonia a critical part in the manufacture of plant fertilizers. The reaction between nitrogen gas and hydrogen gas to produce ammonia gas is exothermic releasing 924kJmol of energy at 298K 25oC.
The Haber Process N2g 3H2-1 Note. To maximise our production of ammonia we could try. Nitrogen can be separated out by fractional distillation.
2021-01-18 The Haber Bosch process which is also called the Haber process is basically one of the most successful and efficient industrial procedures for ammonia productionproduction. 2002-09-01 PDF On Sep 1 2002 Jayant M. Some notes on the conditions.
This development followed by its scaling up to an industrial process by Carl Bosch helped German preparations for the First World War which started in 1914. A brief summary of the Haber Process. The reaction is reversible and the production of ammonia is exothermic.
This process produces an ammonia NH 3 g yield of approximately 10-20. Of process engineering as static in favor of a more dynamic approach. The Haber process is reduced at higher temperatures using Le Chateliers principle explain why the Haber process is based on a delicate balancing act involving reaction energy reaction rate and equilibrium Analyse the impact of increased pressure on the system involved in the.
It was originally developed by German Physical chemist Fritz Haber the method was then improved by Carl Bosch a German chemist and engineer who took the method to an industrial-scale process by using a catalyst. Increasing the pressure we have 4 moles of gas on the left 2 on the right so if we increase the pressure the system will try to lower its pressure by shifting the. Instead of simple yield in a once-through process he concen-trated on space-time yield in a system with recycle.
BASF purchased Habers patents and started develop-. It combines nitrogen from the air with hydrogen that is mainly from natural gas to create ammonia. In the 20th century a German chemist named Fritz Haber and his assistant developed the Haber process catalyst and high-pressure devices to carry out this process in a laboratory.
N 2 g nitrogen 3H 2 g hydrogen heat pressure catalyst 2NH 3 g. Haber Process for Ammonia Synthesis Introduction Fixed nitrogen from the air is the major ingredient of fertilizers which makes intensive food production possible. The Haber process now produces about half of all the nitrogen used.
The Haber Process combines nitrogen from the air with hydrogen derived mainly from natural gas methane into ammonia. 2020-08-15 The Haber Process is used in the manufacturing of ammonia from nitrogen and hydrogen and then goes on to explain the reasons for the conditions used in the process. The Haber process comes from methane from natural gas.
Is the Haber Process still important today. The Haber process as we now call it is still used to make ammonia and helps feed the world by providing the starting. The process was developed in the early 1900s by Fritz Haber and was later modified to become an industrial process to make fertilizers by Carl Bosch.
CH4g H2Og COg 3H2g The nitrogen gas needed for the Haber process comes from the air. Adoption of the process. The Haber Process is the industrial method to produce Ammonia.
A flow scheme for the Haber Process looks like this. The reaction is reversible and the production of ammonia is exothermic. During the devel-opment of inexpensive nitrogen fixation processes many principles of chemical and high-pressure processes were clarified and the field of chemical engineering emerged.
Modak published Haber process for ammonia synthesis Find read and cite all the research you need on ResearchGate. The air is 78 nitrogen. The ammonia produced was oxidised for the production of nitric acid in the Ostwald process and the nitric acid for the production of various explosive nitro compounds used in munitions.
The Haber synthesis was developed into an industrial process by Carl Bosch.
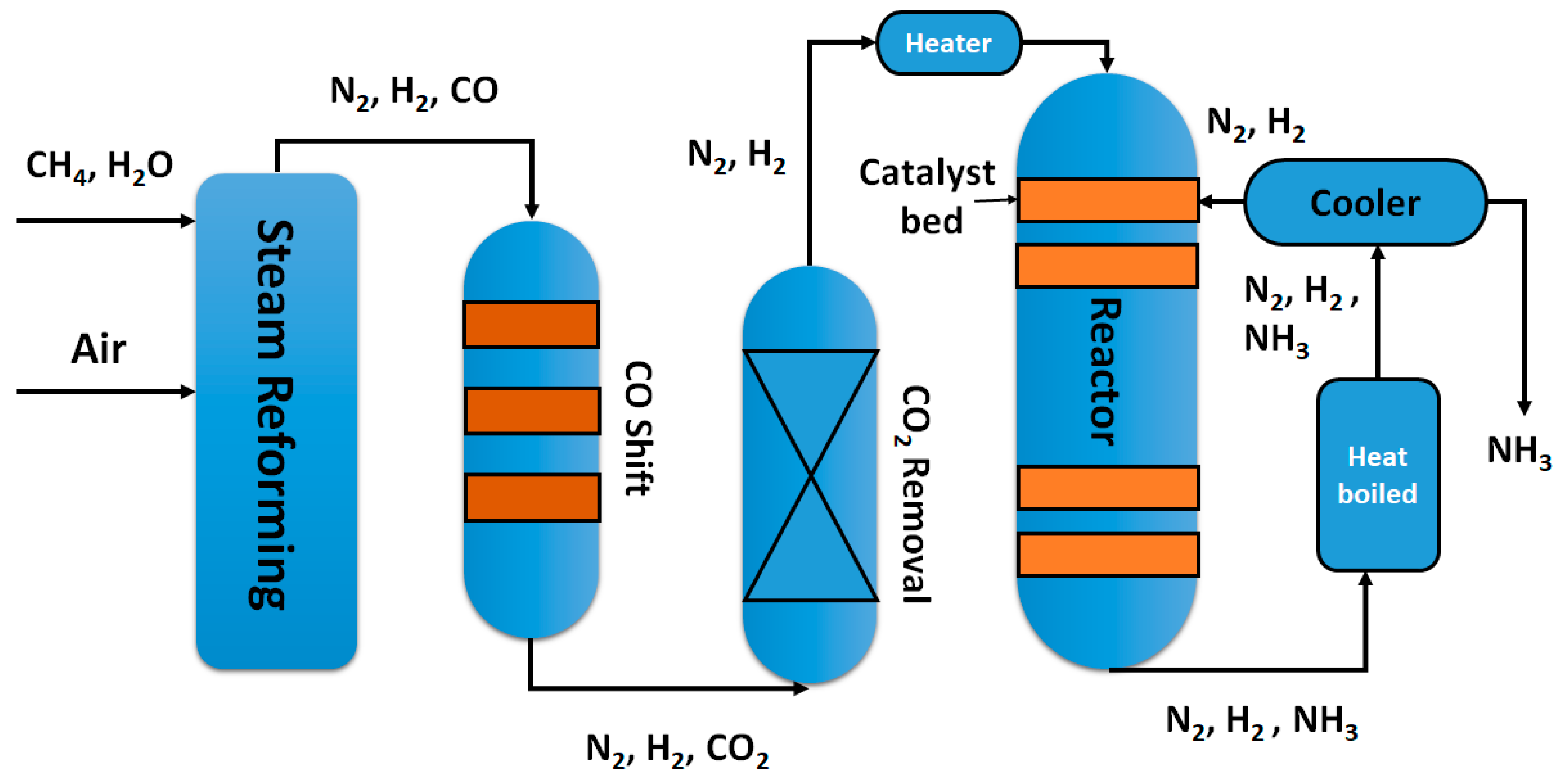 Catalysts Free Full Text Insights Into The Recent Progress And Advanced Materials For Photocatalytic Nitrogen Fixation For Ammonia Nh3 Production Html
Catalysts Free Full Text Insights Into The Recent Progress And Advanced Materials For Photocatalytic Nitrogen Fixation For Ammonia Nh3 Production Html
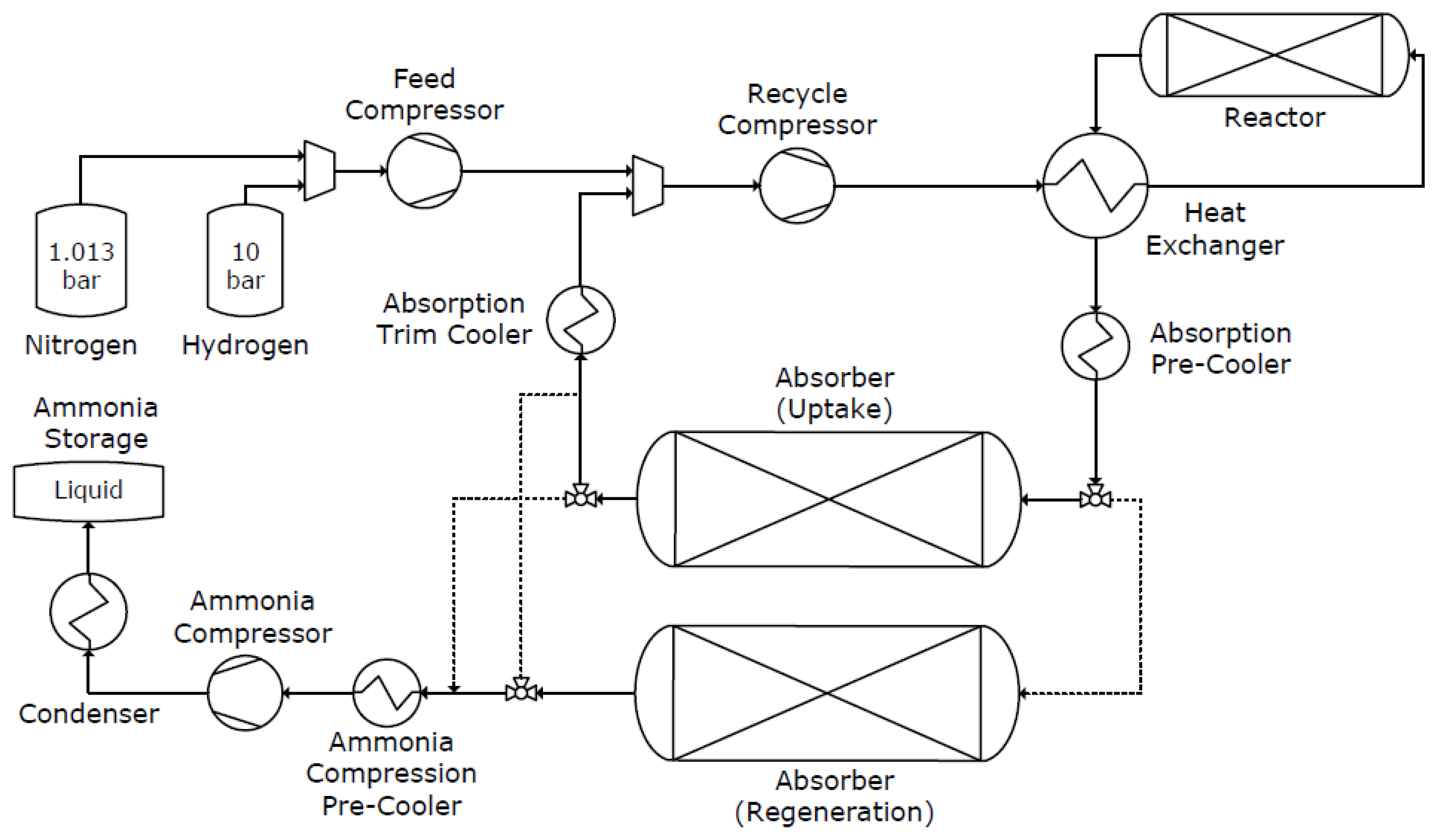 Processes Free Full Text Modeling And Optimal Design Of Absorbent Enhanced Ammonia Synthesis Html
Processes Free Full Text Modeling And Optimal Design Of Absorbent Enhanced Ammonia Synthesis Html
Https Www Chem Tamu Edu Rgroup Marcetta Chem362 Hw 2017 20student 20posters Nitrogenase 20nitrogen 20fixation 20vs 20haber Bosch 20process Pdf
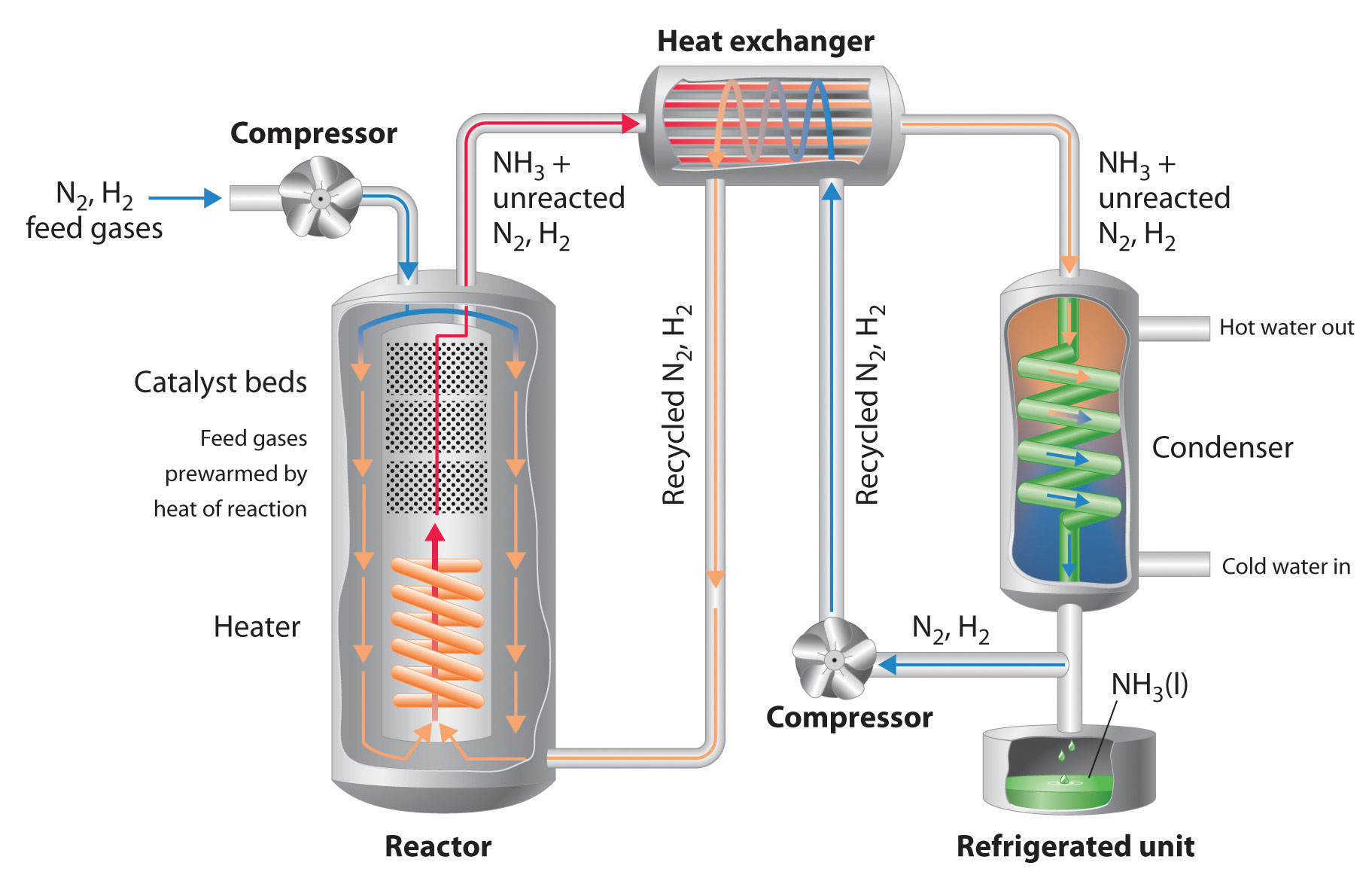 Chapter 14 6 Controlling The Products Of Reactions Chemistry Libretexts
Chapter 14 6 Controlling The Products Of Reactions Chemistry Libretexts