Write The Balanced Chemical Equation For The Haber Bosch Process
Phase symbols are optional. It is named after its inventors the German chemists Fritz Haber and Carl Bosch who developed it in the first decade of the 20th century.
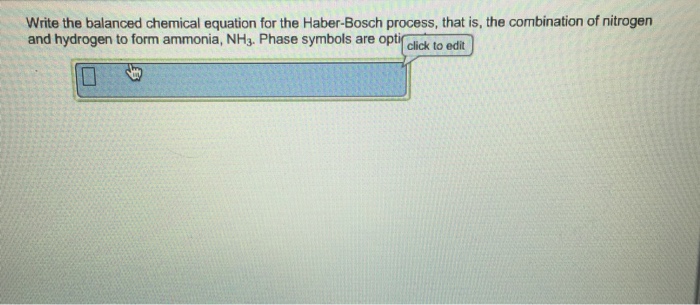
CeN2g 3H2g.
Write the balanced chemical equation for the haber bosch process. The Haber Process again The equilibrium constant for the reaction of nitrogen and hydrogen to give ammonia is 0118 at 745 K. Phase symbols are optional. 2013-04-17 Write a balanced chemical equation for nitrogen gas reacts with oxygen gas to produce nitrogen dioxide gas.
Hydrogen is a very important gas in the chemical industry and can be produced industrially through a number of methods. It can be produced industrially basically by three methods. The process converts atmospheric nitrogen to ammonia by a.
2020-08-15 The process combines nitrogen from the air with hydrogen derived mainly from natural gas methane into ammonia. N2g 3H2g2NH3g 2. The reaction is reversible and the production of ammonia is exothermic.
The haber process This page describes the Haber Process for the manufacture of ammonia from nitrogen and hydrogen and then goes on to explain the reasons for the conditions used in the process. 3H2 N2. 2001-10-29 The Haber process also called the HaberBosch process is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today.
It looks at the effect of temperature pressure and catalyst on the composition of the equilibrium mixture the rate of the reaction and the economics of the process. The Haber-Bosch process played a significant role in boosting agriculture back in the day. I Bosch processes ii From Methane and iii Through the Electrolysis of brineas a by-product.
Write the balanced chemical equation for the Haber-Bosch process that is the combination of nitrogen and. CO 2 g 2 H 2 g C s 2 H 2 O g The above. As we know that one mole of N2reacts with 3 moles of H2to form 2 moles of NH3.
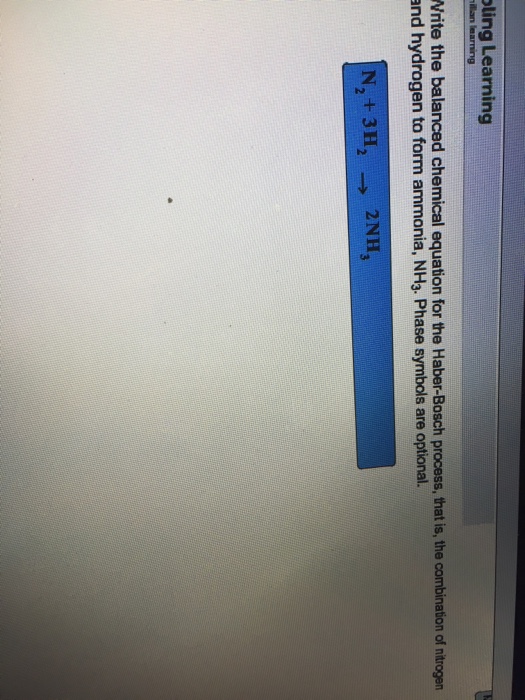
The balanced chemical equation for the Haber-Bosch process is N₂ g 3H₂ g 2NH₃ g. 2NH3g nonumber What is K_p for this reaction at the same temperature. Phase symbols are optional.
It paved the way for the industrial production of ammonia which is used in the manufacture of fertilizers. 2013-11-24 write the balanced chemical equation for the haber-bosch process that is the combination of hydrogen and nitrogen to form ammonia NH3-. Bosch process is used in the industrial preparation of Hydrogen.
Separate the following balanced chemical equation into its total ionic equation. Nh3 is used as fertilizer. 2NH3 g label eq1 with ΔH-924 kJmol.
It is oxidized with O2 to make HNO3. 2015-09-20 Write the balanced chemical equation for the Haber-Bosch process that is the combination of nitrogen and hydrogen to form ammonia NH3. As we kn view the full answer.
Ce N2 g 3H2 g. Write the balanced chemical equation for the Haber-Bosch process that is the combination of nitrogen and hydrogen to form ammonia NH3. German chemists Fritz Haber along with his assistant in the 20th century developed high-pressure devices and catalysts to carry out the process on a laboratory scale.
Write the balanced chemical equation for the Haber-Bosch process that is the combination of nitrogen and hydrogen to form ammonia NHeq_3eq. Solution for Write the balanced chemical equation for the HaberBosch process ie the combination of nitrogen and hydrogen to form ammonia NH3NH3. This reaction requires the introduction of iron as a catalyst and requires a temperature level of 530-730 degrees Celsius.
Hence The balanced chemical equation for Haber-Bosch process is as follows. NaNO3 aqAgCls List the ions in order of the above equation. It is named after the German chemist Carl Bosch.
I do not know what is the typical. HaberBosch process or just Haber process is basically one of the most efficient and successful industrial procedures to be adopted for the production of ammonia. The overall reaction is as follows.
Write the balanced equation for the following by inserting the correct coefficients in the blanks___ C4H10 g ___ O2 g ___ H2O l ___ CO2 g Problem. The balanced equilibrium equation is as follows.

 Write The Balanced Chemical Equation For T Clutch Prep
Write The Balanced Chemical Equation For T Clutch Prep
 Solved Write The Balanced Chemical Equation For The Haber Chegg Com
Solved Write The Balanced Chemical Equation For The Haber Chegg Com
 Solved Write The Balanced Chemical Equation For The Haber Chegg Com
Solved Write The Balanced Chemical Equation For The Haber Chegg Com