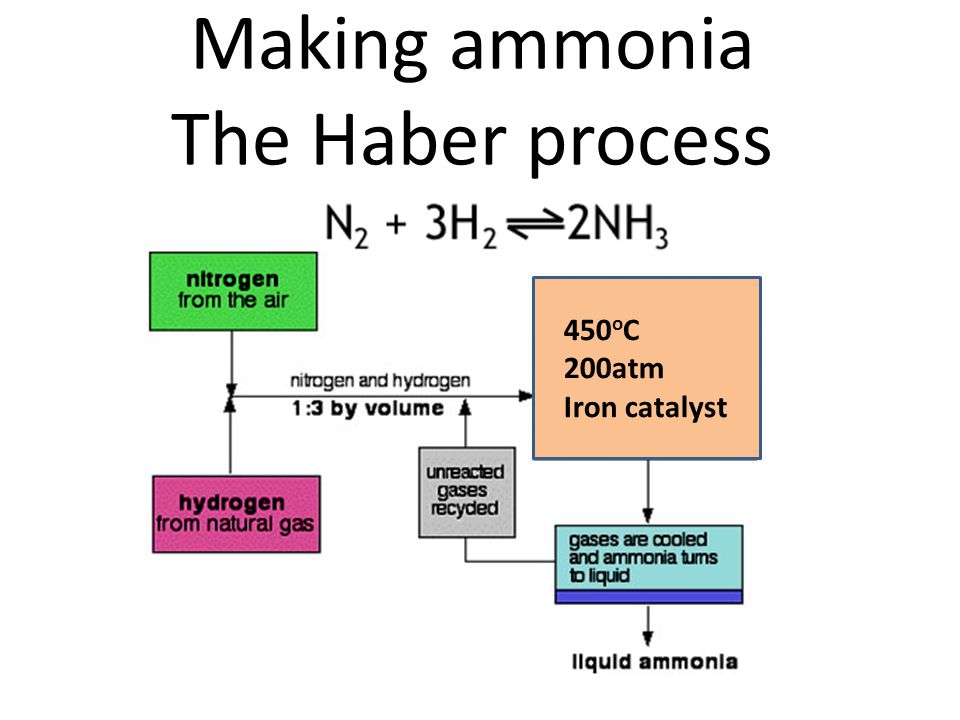
Conditions Used In The Haber Process
Increasing the pressure of. You must also be able to USE the ideas on other unfamiliar equilibria.
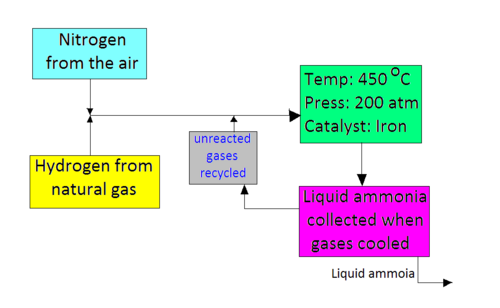 The Haber Process Wikieducator
The Haber Process Wikieducator
EFFECT ON THE POSITION OF EQUILIBRIUM.
Conditions used in the haber process. It directly combines nitrogen from the air with hydrogen under extremely high pressures and moderately high temperatures. 2021-01-18 In this process a metal catalyst is used and high pressures and temperatures are maintained. Haber-Bosch was the first industrial chemical process to use high pressure for a chemical reaction.
Iron is the catalyst and does not get used up in the reaction. 2020-08-15 The Haber Process is used in the manufacturing of ammonia from nitrogen and hydrogen and then goes on to explain the reasons for the conditions used in the process. The forward reaction is exothermic while the reverse reaction is exothermic while the reverse reaction is endothermic.
These details and conditions need to be remembered. EFFECT ON THE POSITION OF EQUILIBRIUM. The Haber Process is the process by which ammonia NH 3 is produced.
Nitrogen extracted from the air and hydrogen obtained from natural gas are pumped through pipes a compressor increases the gas pressure to. Air which supplies the nitrogen. You must also be able to USE the ideas on other unfamiliar equilibria.
The symbol you see in the middle means it is a reversible reaction so the product can decompose back into the reactants. Optimum conditions must be selected to. The reaction is reversible and the production of ammonia is exothermic.
The equation for this reaction is. Natural gas and water supply the hydrogen and the energy needed to heat the reactants. The forward reaction of the Haber process is exothermic heat energy released therefore the forward reaction will favour a low temperature.
The Haber process is an important industrial process which needs to be understood for A-level. So in the context of the Haber process the conditions which can be altered are temperature and pressure. The raw materials that are used for the process are listed below.
It looks at the effect of temperature pressure and catalyst on the composition of the equilibrium mixture the rate of the reaction and the economics of the process. The forward direction is exothermic -ve enthalpy change value. 2019-04-10 The Haber-Bosch process uses a catalyst or container made of iron or ruthenium with an inside temperature of over 800 F 426 C and a pressure of around 200 atmospheres to force nitrogen and hydrogen together Rae-Dupree 2011.
The process combines nitrogen from the air with hydrogen derived mainly from natural gas methane into ammonia. 2020-12-31 In the Haber Process which is deployed in the commercial or industrial production of ammonia every 3 moles of hydrogen gas will react with 1 mole of nitrogen gas to yield 2 moles of ammonia. In the Haber process.
2017-12-12 The Haber process involves an equilibrium reaction and knowledge of Le Chateliers principle is needed in order to predict how reaction conditions will impact on the production of ammonia by this process Rule 1. Due to the Haber process being a reversible reaction the yield of ammonia can be changed by changing the pressure or temperature of the reaction. These details and conditions need to be remembered.
The Haber process is an important industrial process which needs to be understood for A-level. This page describes the Haber Process for the manufacture of ammonia from nitrogen and hydrogen and then goes on to explain the reasons for the conditions used in the process.
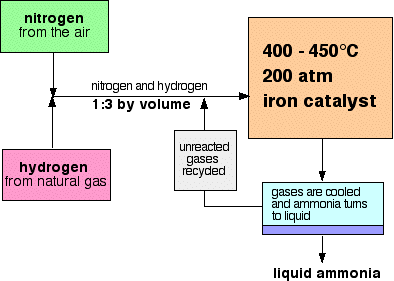 The Haber Process For The Manufacture Of Ammonia Chemkey
The Haber Process For The Manufacture Of Ammonia Chemkey