How Does The Haber Process Affect The Environment
Using a lower temperature though would make the reaction slower and knowing that temperature increases the rate of the reaction 400-450 degress celsius is used instead. 2019-04-10 The Haber-Bosch process is a process that fixes nitrogen with hydrogen to produce ammonia a critical part in the manufacture of plant fertilizers.
 The Haber Process Daniel D Dulek Ap Environmental Science Chemical Reactions Ap Chem
The Haber Process Daniel D Dulek Ap Environmental Science Chemical Reactions Ap Chem
Though it has increased food supply worldwide the Haber process has also taken an unforeseen toll on the environment.

How does the haber process affect the environment. Very little if anything. It will cause a gap in the food chain and will alter things so that it will do nothing but damage. The Haber Process for the synthesis of ammonia NH3 gas from its elements nitrogen N2 and hydrogen H2 is discussed in almost every high school chemistry text as an excellent example of chemical equilibrium.
The position of the equilibrium and the rate of reaction. Stay tuned with BYJUS to learn more. 2013-05-08 Regarding the environment it affects it directly when the animals that are have a habitat in that area are killed it will directly or indirectly effect the environment.
2001-10-29 Nitrogen gas N 2 is very unreactive because the atoms are held together by strong triple bonds. It also contributes to a build-up of reactive nitrogen in the biosphere causing potential harm to the nitrogen cycle. The most important factor in deciding what conditions to use is therefore not yield but total cost.
In 1910 the German chemist Fritz Haber discovered a process to convert atmospheric nitrogen into ammonia a workable form of nitrogen using high temperature and high pressure machines. How Does Haber Process Affect The Environment How Does Haber Process Affect The Environment. This is a compromise since it results in a lower yield but a much faster process.
Production of fertilizer-N via the Haber-Bosch process consumes an enormous amount of fossil fuel. 2015-01-01 Today agriculture uses over 100 million metric tons of fertilizer-N each year which has doubled the flux of N through the terrestrial N-cycle. The Haber compromise To produce a high yield of ammonia but with a fast rate of reaction and without the need for overly expensive equipment the Haber process is carried out at 450C and 200 atmospheres.
The Haber process relies on catalysts that accelerate the scission of this triple bond. Dulek delves into the chemistry and consequences. A brief summary of the Haber Process The Haber Process combines nitrogen from the air with hydrogen derived mainly from natural gas methane into ammonia.
2020-06-01 For example the Haber Process uses tonnes of energy consuming around 1-2 of the world energys supply. A flow scheme for the Haber Process looks like this. Two opposing considerations are relevant to this synthesis.
2016-10-06 Because Habers process releases a lot of heat ideally a lower temperature would be used. Eutrophication and biodiversity loss are the side effects of the Haber process to the environment. The process was developed in the early 1900s by Fritz Haber and was later modified to become an industrial process to make fertilizers by Carl Bosch.
2016-03-24 The Haber process named after Fritz Haber is a method of synthesizing ammonia NH3 see diagrams below from nitrogen out of the air and hydrogen from natural gas using iron as a catalyst in an environment of high temperature and pressure. The reaction is reversible and the production of ammonia is exothermic. Raw materials equipment energy.
The Haber-Bosch process is considered by many scientists and. The process revolutionized mass agriculture across the world and its first major use was in the BASF labs in Ludwigshafen Germany in 1913. 2013-11-18 Its called the Haber process which turns the nitrogen in the air into ammonia easily converted in soil to the nitrate plants need to survive.
 What Is The Haber Bosch Process And Its Ecological And Human Impact
What Is The Haber Bosch Process And Its Ecological And Human Impact
 The Haber Process Environmental Challenges Crop Yields Nitrogen
The Haber Process Environmental Challenges Crop Yields Nitrogen
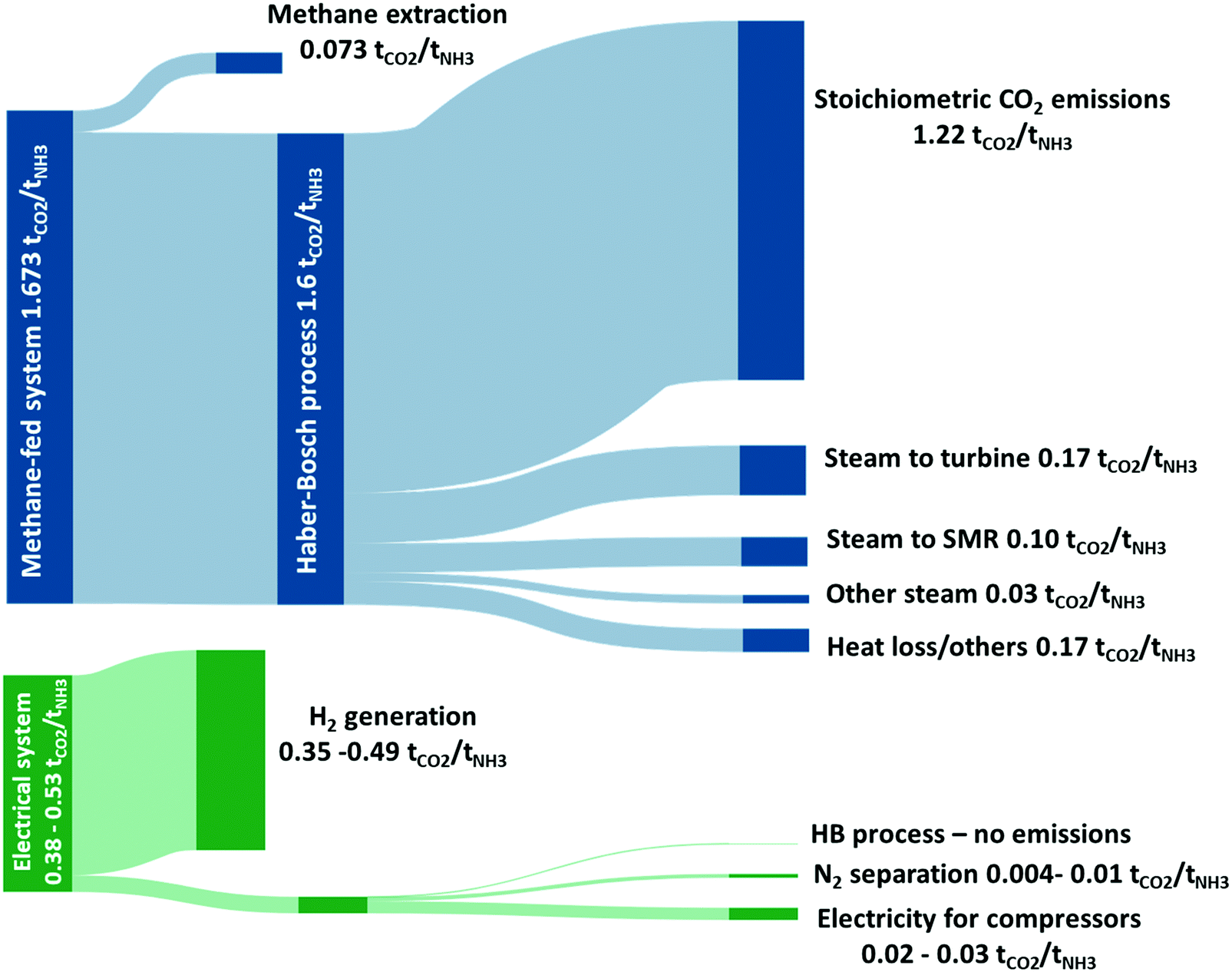 Current And Future Role Of Haber Bosch Ammonia In A Carbon Free Energy Landscape Energy Environmental Science Rsc Publishing Doi 10 1039 C9ee02873k
Current And Future Role Of Haber Bosch Ammonia In A Carbon Free Energy Landscape Energy Environmental Science Rsc Publishing Doi 10 1039 C9ee02873k
 5 Key Findings About Public Trust In Scientists In The U S Pew Research Center Social Science Research History Of Science Content Analysis
5 Key Findings About Public Trust In Scientists In The U S Pew Research Center Social Science Research History Of Science Content Analysis