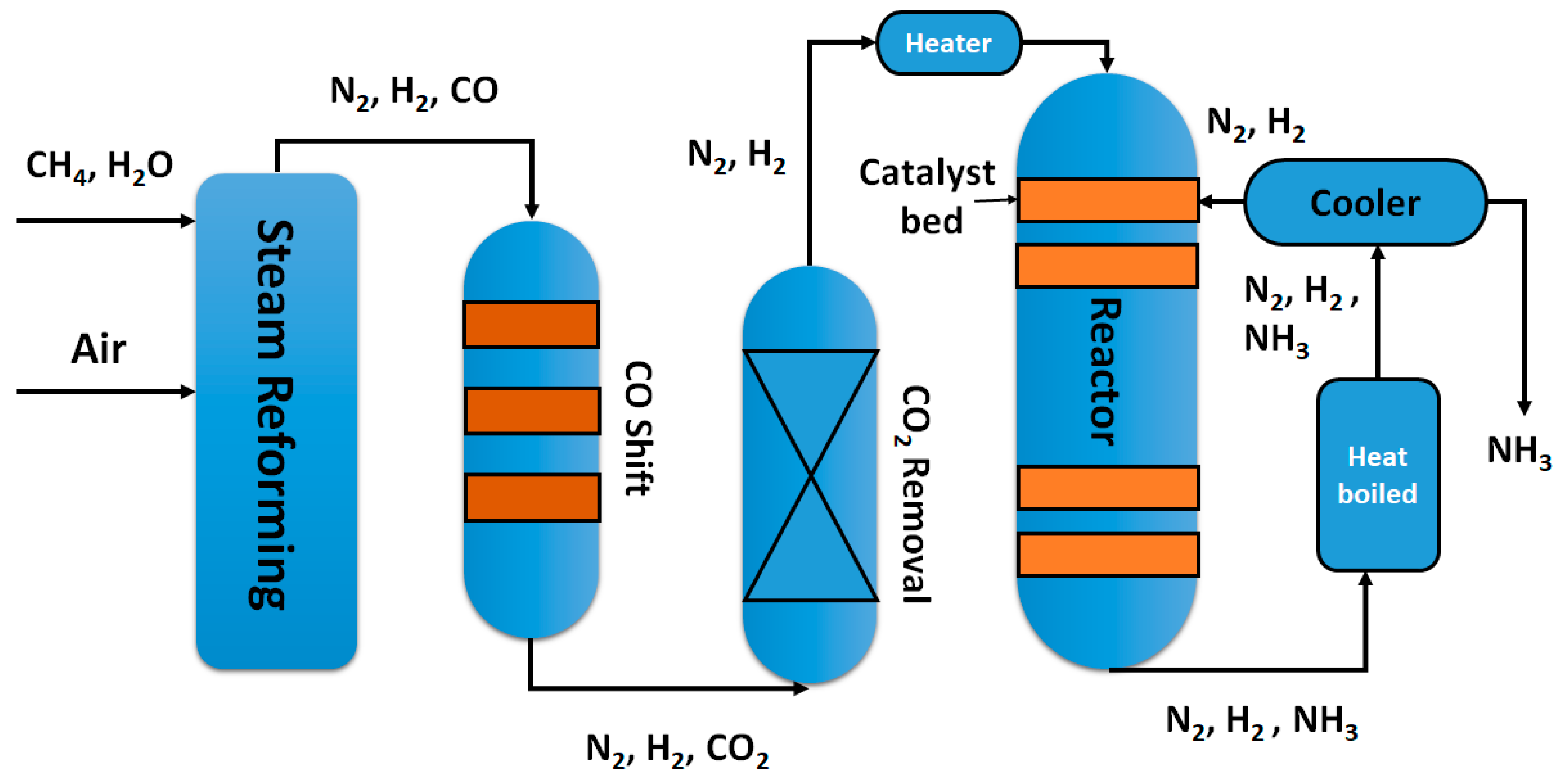
Haber Bosch Process Steps
N 2 3H 2 2NH 3. Having been scrubbed both gasses are mixed and the mixture is piped into a compressor.
 Catalysts Free Full Text Insights Into The Recent Progress And Advanced Materials For Photocatalytic Nitrogen Fixation For Ammonia Nh3 Production Html
Catalysts Free Full Text Insights Into The Recent Progress And Advanced Materials For Photocatalytic Nitrogen Fixation For Ammonia Nh3 Production Html
Are the net result of the elementary steps involved in the mechanism and there is often no single chemical process that corresponds to the reaction equation.
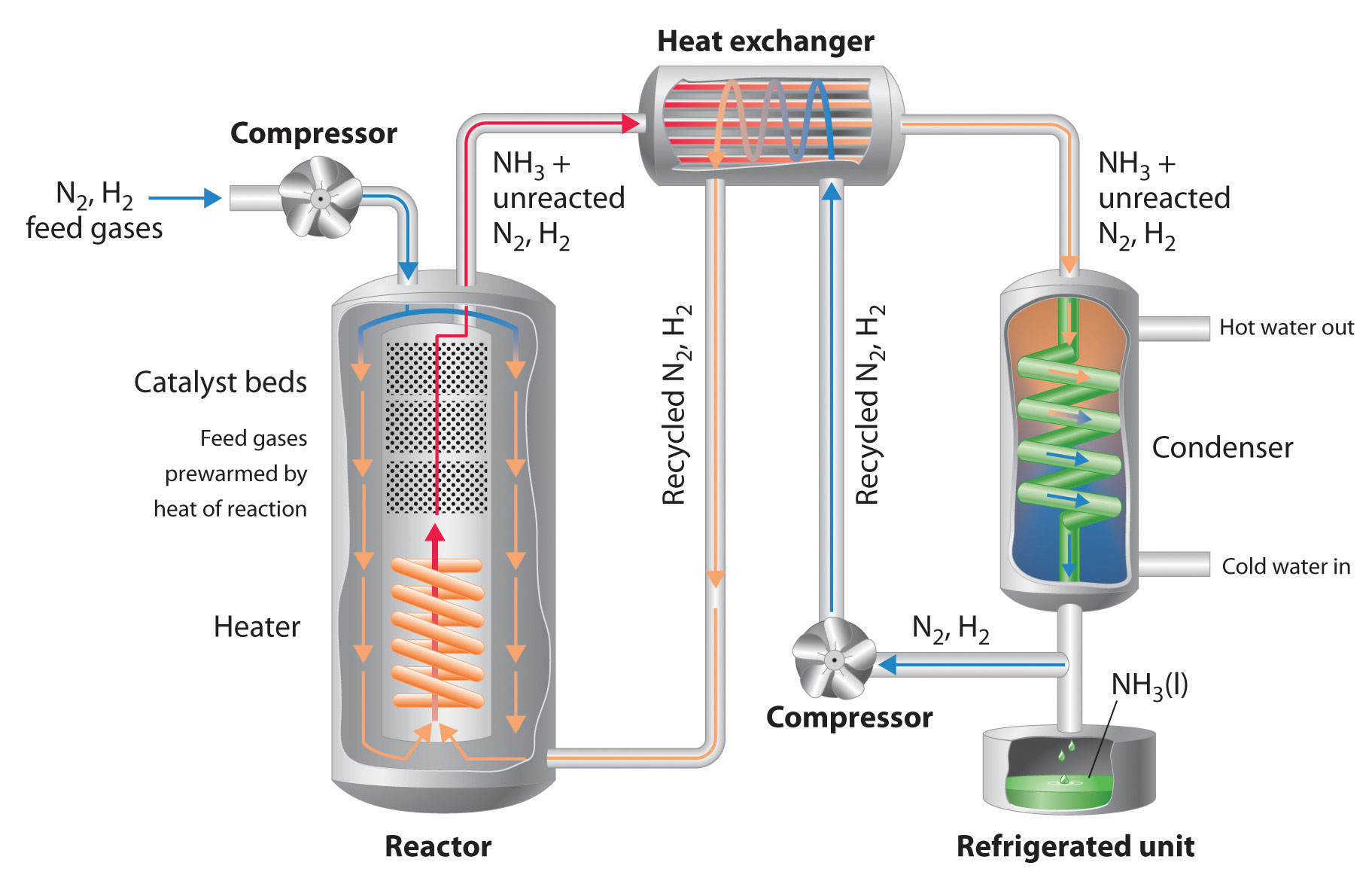
Haber bosch process steps. The compressed mixture of gas. Since ammonia condenses easily as compared to nitrogen and hydrogen the liquefied ammonia is collected and removed and the leftover nitrogen and hydrogen gases are re-introduced into the reactor. The Haber process reacts nitrogen and hydrogen gas to form ammonia.
3 H 2 N 2. 2019-04-10 The Haber-Bosch process is a process that fixes nitrogen with hydrogen to produce ammonia a critical part in the manufacture of plant fertilizers. The reaction is reversible and the production of ammonia is exothermic.
Reacted with nitrogen derived from process air to form anhydrous liquid ammonia. 1 Transport of the reactants by diffusion and convection out of the bulk gas stream through a laminar boundary layer to the outer surface of the catalyst particles and further through the pore system to the inner surface pore walls. 2020-08-15 The Haber Process is used in the manufacturing of ammonia from nitrogen and hydrogen and then goes on to explain the reasons for the conditions used in the process.
Here the mixture is compressed. The process was developed in the early 1900s by Fritz Haber and was later modified to become an industrial process to make fertilizers by Carl Bosch. 2011-08-25 The Process- The Steps The two raw materials for the Reaction are obtained-nitrogen and hydrogen.
They undergo a process known as scrubbing. For example many readers will be familiar with the well-known HaberBosch process for producing ammonia NH 3 from molecular nitrogen and hydrogen. The overall reaction equation is.
The reaction is reversible and the production of ammonia is exothermic. A flow scheme for the Haber Process looks like this. N 2 adsorbed 3H 2 adsorbed NH 3 adsorbed The hot mixture of gases is then passed through a condenser.
Producing ammonia is by the Haber-Bosch process which involves the direct reaction of elemental hydrogen and elemental nitrogen. The Haber-Bosch process synthesizes ammonia from atmospheric nitrogen and hydrogen under high temperatures and pressures for use in artificial fertilizers and. This step is known as the ammonia synthesis loop also referred to as the Haber-Bosch process.
N 2 3 H 2 2 NH 3 ΔH 924 kJmol 1 History of the Haber Process. A simplified schematic of the methane-fed HaberBosch process is depicted in Fig. The first is hydrogen production from methane and the second is ammonia synthesis by the HaberBosch reaction.
A brief summary of the Haber Process The Haber Process combines nitrogen from the air with hydrogen derived mainly from natural gas methane into ammonia. Trailer for The Haber-Bosch Process121 Honors Chemistry Project By. The process combines nitrogen from the air with hydrogen derived mainly from natural gas methane into ammonia.
2019-02-01 The Haber process or Haber-Bosch process is the primary industrial method used to make ammonia or fix nitrogen. A modern ammonia manufacturing process is highly integrated but can be broken down into two main functional steps. The ammonia synthesis by the Haber-Bosch process as every catalytic gas-phase reaction can be divided into the following steps.
The Haber-Bosch process is considered by many scientists and.
 Haber Bosch Process An Overview Sciencedirect Topics
Haber Bosch Process An Overview Sciencedirect Topics
 A Simple Explanation Of Haber Bosch Process For Ammonia Production Science Struck
A Simple Explanation Of Haber Bosch Process For Ammonia Production Science Struck
The Haber Bosch Process The Haber Process Sjii
 Chapter 14 6 Controlling The Products Of Reactions Chemistry Libretexts
Chapter 14 6 Controlling The Products Of Reactions Chemistry Libretexts