How Does The Haber Process Work
The process combines nitrogen from the air with hydrogen derived mainly from natural gas methane into ammonia. Hydrogen is obtained by reacting natural gas.
What Type Of Reaction Takes Place In The Haber Process Exothermic Or Endothermic Quora
The Haber process The raw materials for the process of making ammonia are hydrogen and nitrogen.
How does the haber process work. See link below for a discussion of the process. He received the Nobel Prize for Chemistry in 1918 for this method which made the manufacture of ammonia economically feasible. Furthermore in order to overcome the low conversion-per-pass of ammonia he introduced an important concept.
Developed by industrial chemist Fritz Haber and scaled up by the chemical engineer Carl Bosch the Haber-Bosch process takes nitrogen from the air and converts it to ammonia. 2019-04-10 How the Haber-Bosch Process Works. Nitrogen extracted from the air and hydrogen obtained from natural gas are pumped through pipes the pressure of the mixture of.
Increasing the temperature will make the reaction faster but will shift the equilibrium to make less products. Haber first proposed the use of a high-pressure reaction technique. This made it possible for the first time to produce synthetic fertilisers and produce sufficient food for the Earths growing population.
The Haber process is an exothermic reaction that happens in equilibrium which creates the following problem. Haber-Bosch process also called Haber ammonia process or synthetic ammonia process method of directly synthesizing ammonia from hydrogen and nitrogen developed by the German physical chemist Fritz Haber. The Haber process reacts nitrogen and hydrogen gas to form ammonia.
2019-02-01 The Haber process or Haber-Bosch process is the primary industrial method used to make ammonia or fix nitrogen. The reaction is reversible and the production of ammonia is exothermic. 2017-01-02 A hundred years ago two German chemists Fritz Haber and Carl Bosch devised a way to transform nitrogen in the air into fertiliser using what became known as the Haber-Bosch process.
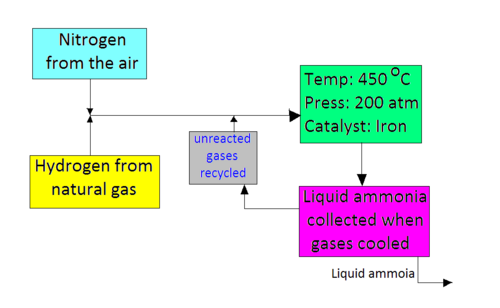
Nitrogen which reacts very little at normal temperatures and pressures is placed under heat and pressure and is. N 2 3 H 2 2 NH 3 ΔH 924 kJmol 1 History of the Haber Process. The process must use high pressure because nitrogen molecules are held together with strong triple bonds.
The Haber Process combines nitrogen from the air with hydrogen derived mainly from natural gas methane into ammonia. The Haber process is the process that uses extracted nitrogen from the atmosphere and reacts the nitrogen N2 gas would react with 3 moles of hydrogen H2 gas by using a medium temperature around 473K-673K 200- 400C High atmospheric pressures such as 250 atmospheres 25331250 Pascal and a catalyst to create. It works by fixing nitrogen from the air with hydrogen from natural gas to produce ammonia.
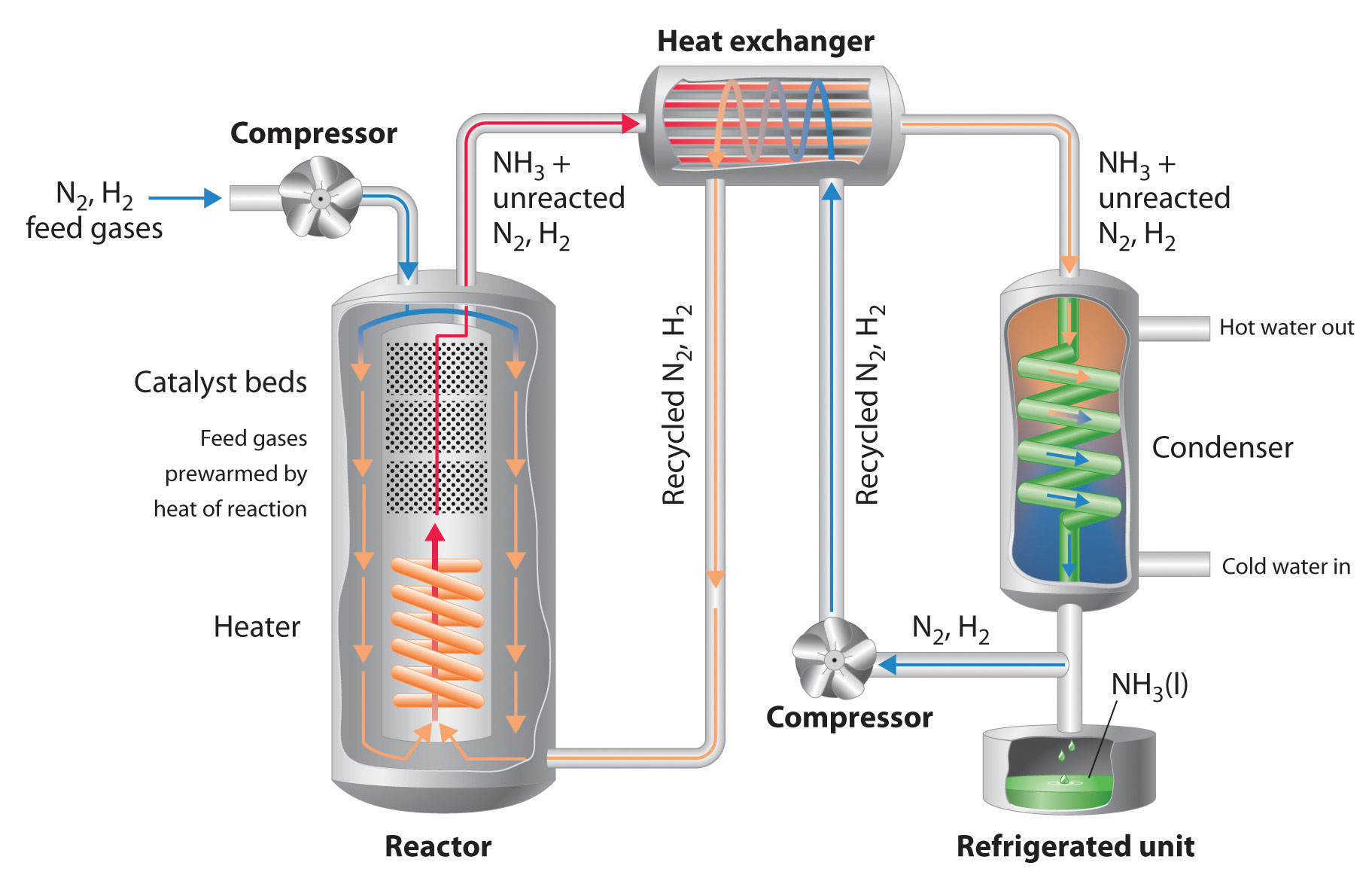
2020-08-15 The Haber Process is used in the manufacturing of ammonia from nitrogen and hydrogen and then goes on to explain the reasons for the conditions used in the process. The process works today much like it originally did by using extremely high pressure to force a chemical reaction. Firstly Nitrogen and Hydrogen the raw materials are scrubbed or cleaned to remove any impurities.
A flow scheme for the Haber Process looks like this. 2016-03-24 The Haber process named after Fritz Haber is a method of synthesizing ammonia NH3 see diagrams below from nitrogen out of the air and hydrogen from natural gas using iron as a catalyst in an environment of high temperature and pressure. In the Haber process.
The reaction rate which is used in space-time yield to replace reaction yield. Some notes on the conditions. The mixture of Hydrogen and Nitrogen is compressed until the pressure reaches 200 atmospheres.
 The Haber Process Wikieducator
The Haber Process Wikieducator
 Synthesis Of Ammonia Process Reaction Science Class 2021 Video Study Com
Synthesis Of Ammonia Process Reaction Science Class 2021 Video Study Com
 Chapter 14 6 Controlling The Products Of Reactions Chemistry Libretexts
Chapter 14 6 Controlling The Products Of Reactions Chemistry Libretexts